
BEPREVE® demonstrated significant reductions in ocular itching1†‡
Ocular itching score at 3 minutes post CAC at onset-of-action visit (both eyes averaged)
(co-primary endpoint)2,3
Onset-of-action visit required 15 minutes between investigation product dosing and CAC test.
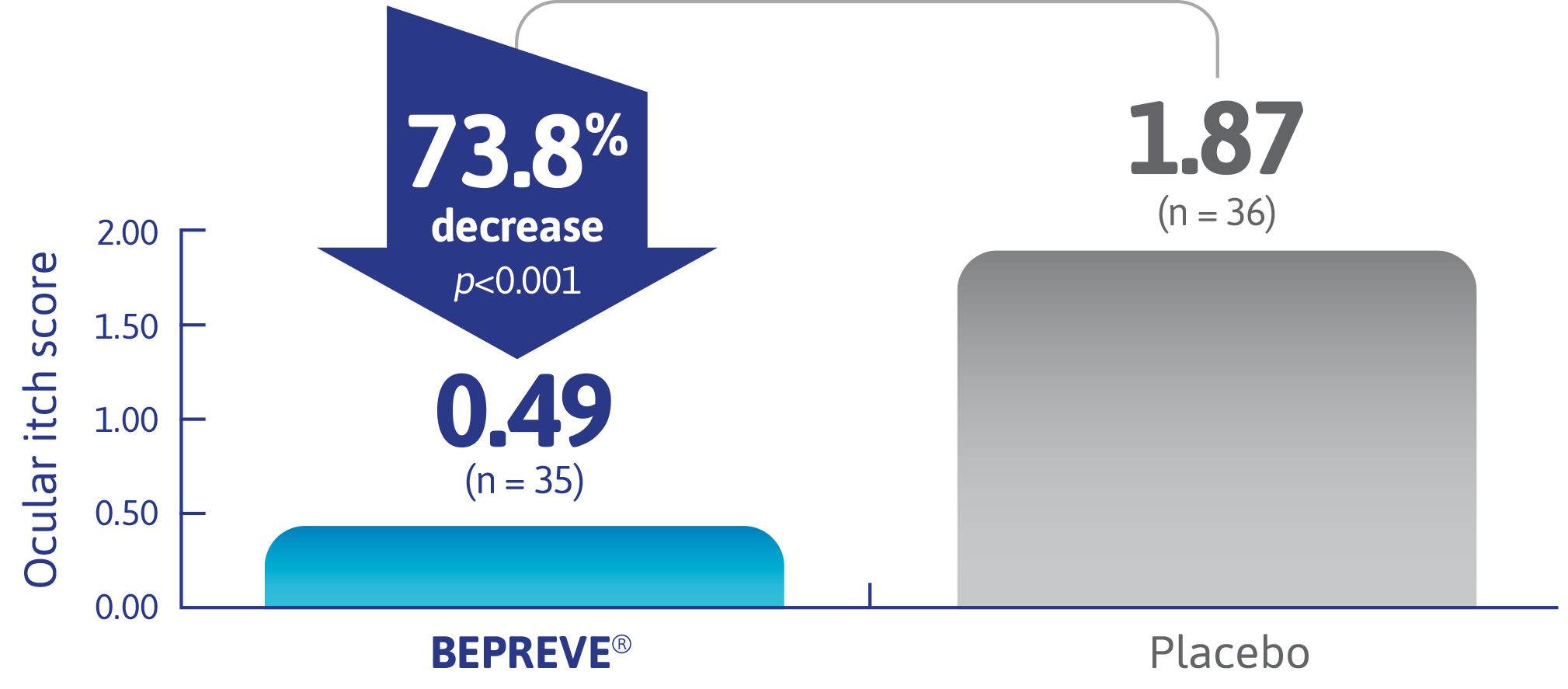
Adapted from Abelson et al.2,3
Mean difference vs. placebo in mean ocular itching score at 3 minutes post CAC
(co-primary endpoint)1
BEPREVE® significantly reduced ocular itch 3 minutes after CAC challenge at visit 4 (8 hours between investigational product dosing and CAC test) (1.3-unit difference at 3 minutes post CAC; p<0.0001).*
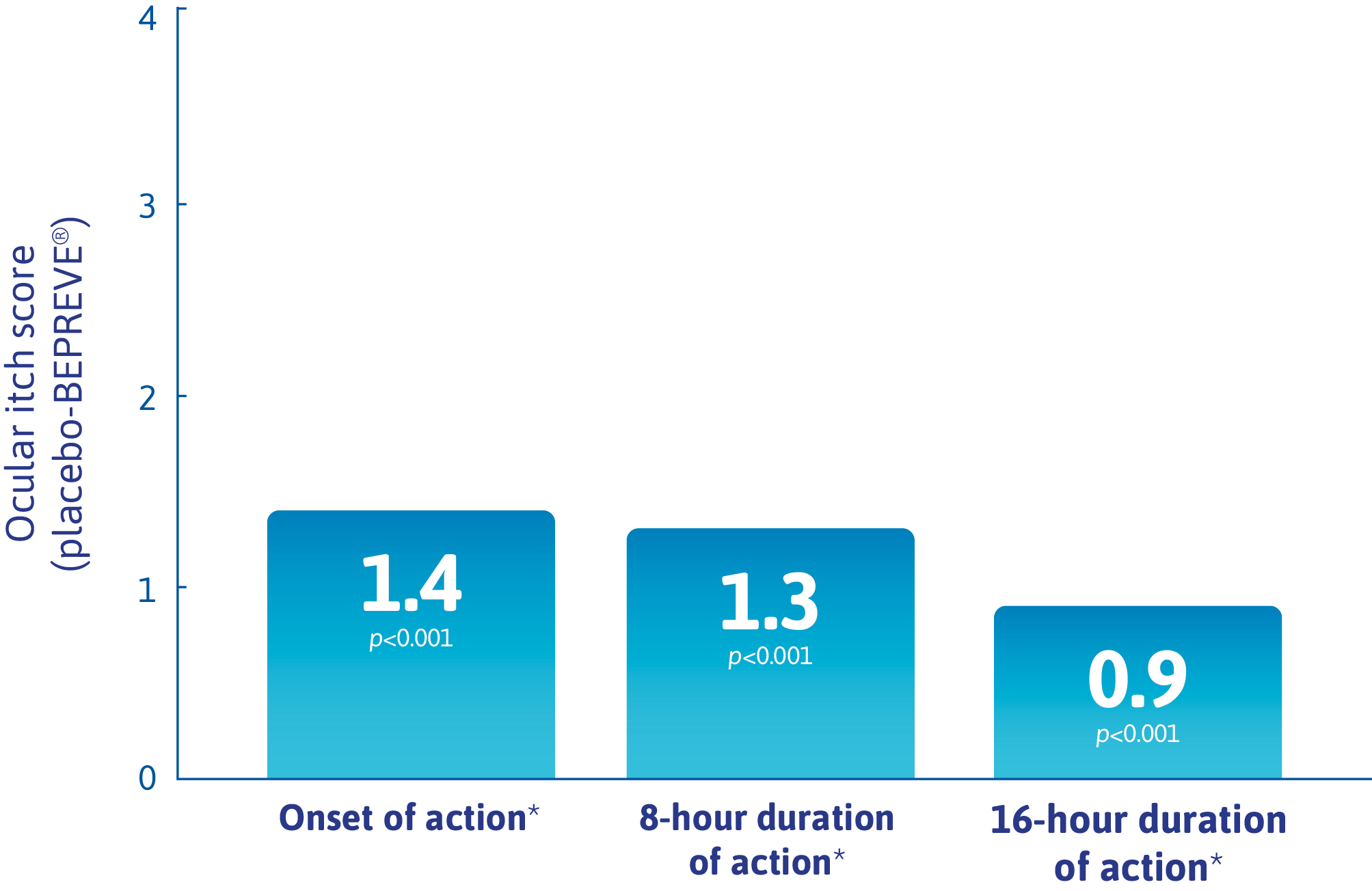
* Visit 3B required 16 hours between investigational product dosing and CAC test.
Visit 4 required 8 hours between investigational product dosing and CAC test.
Visit 5 required 15 minutes between investigational product dosing and CAC test.
Efficacy was established in one Phase 2/3 and one Phase III, placebo-controlled, double-masked, randomized, conjunctival allergen challenge (CAC) clinical trial where participants were randomly assigned to BEPREVE® 1.5% w/v or placebo. CAC testing was done using multiple allergens, including both seasonal and perennial allergens. Study participants included males and females 10 years of age and older who had a positive history of allergic conjunctivitis. Ocular itching (measured on a 5-point scale, from 0 [none] to 4 [incapacitating itch with an irresistible urge to rub]) was evaluated at 3, 5 and 7 minutes following a CAC.1
BEPREVE® demonstrated clinical significance for the reduction of ocular itching in the onset-of-action CAC test starting at 3 minutes, and 8-hour duration-of-action CAC test. Clinical significance was defined as change of more than one unit on the grading scale at a majority of timepoints evaluated.1,2
